Transdermal Capsicum Patches OEM: Ensuring Compliance with Global Regulatory Standards
In the fast-growing market for natural pain management solutions, Transdermal Capsicum Patches stand out as an effective, user-friendly product category. For brands seeking to expand globally, partnering with a trusted Transdermal Capsicum Patches OEM who understands and adheres to international regulatory requirements is vital to ensure product safety, quality, and market access.
This comprehensive guide explores the critical role of regulatory compliance in the manufacturing and distribution of Custom Transdermal Capsicum Patches and Private Label Transdermal Capsicum Patches, outlines key global standards, and highlights how the right Transdermal Capsicum Patches Manufacturer and Supplier can help your brand succeed across markets.
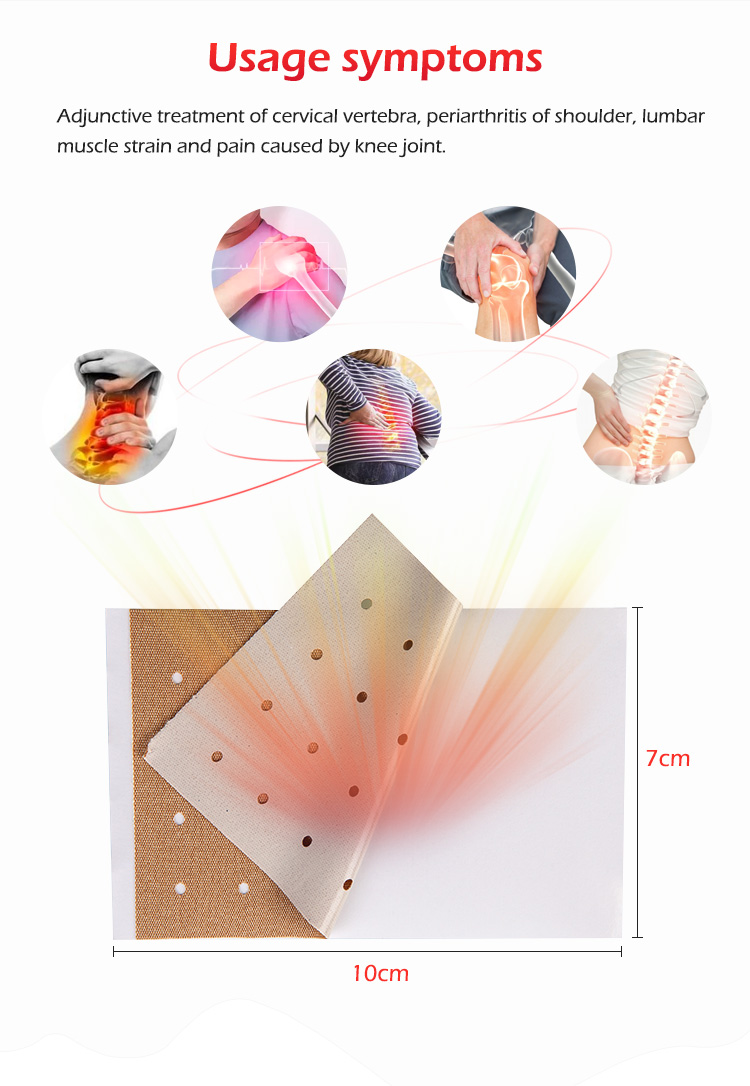
1️⃣ Why Regulatory Compliance Matters in Transdermal Capsicum Patches OEM
Regulatory compliance is the backbone of safe and trustworthy healthcare products. For Transdermal Capsicum Patches, which deliver active compounds like capsaicin through the skin, strict adherence to manufacturing, safety, labeling, and marketing standards is critical.
Key reasons compliance matters:
Consumer Safety: Ensures patches meet safety thresholds for skin irritation, allergenicity, and efficacy.
Legal Market Access: Regulatory approval or certification is required to sell in markets like the US, EU, Canada, and Asia.
Brand Reputation: Compliance reinforces consumer trust and reduces risk of recalls or legal action.
Supply Chain Integrity: Verified processes protect against contamination and substandard raw materials.
Competitive Advantage: Meeting global standards enables access to lucrative international markets.
2️⃣ Understanding Global Regulatory Frameworks for Transdermal Capsicum Patches
?? United States – FDA Regulations
In the US, Transdermal Capsicum Patches are typically regulated as over-the-counter (OTC) drugs or medical devices depending on claims and ingredients. Key FDA requirements include:
Good Manufacturing Practices (GMP): Ensures quality control throughout production.
Labeling Compliance: Clear ingredient lists, directions, warnings, and claims consistent with FDA guidelines.
Ingredient Safety: Capsaicin concentration limits and safety testing.
Product Registration: Depending on classification, product listing with the FDA may be required.
Adverse Event Reporting: Post-market surveillance and reporting obligations.
?? European Union – CE Mark and Cosmetics Regulation
In the EU, depending on intended use, Transdermal Capsicum Patches may be regulated as medical devices or cosmetics:
Medical Device Regulation (MDR): Requires CE marking, risk assessments, clinical evaluation, and post-market monitoring.
Cosmetic Products Regulation: If marketed for cosmetic benefits, compliance with ingredient restrictions, labeling, and safety assessments is mandatory.
Good Manufacturing Practices: Compliance with ISO 13485 quality management standards.
Authorized Representative: A local EU-based representative is often required for non-EU manufacturers.
?? Canada – Health Canada Regulations
Canada regulates these products as natural health products (NHP) or medical devices:
Product Licensing: Requires a Natural Product Number (NPN) or medical device license.
Site Licensing: Manufacturing sites must be licensed and comply with GMP.
Labeling and Advertising: Must comply with the Natural Health Products Regulations.
Safety and Efficacy Evidence: Submission of supporting documentation to Health Canada.
?? Japan – Pharmaceuticals and Medical Devices Agency (PMDA)
Japan classifies such patches based on function:
Quasi-Drugs or Medical Devices: Require approval or certification.
Good Manufacturing Practices: Compliance with Japanese GMP.
Labeling and Safety: Adherence to stringent local standards.
3️⃣ Role of the Transdermal Capsicum Patches OEM in Regulatory Compliance
A reputable Transdermal Capsicum Patches Manufacturer plays a pivotal role in navigating complex regulatory landscapes.
Key responsibilities include:
GMP-Compliant Manufacturing: Ensuring all production stages meet international quality standards.
Product Testing and Validation: Stability, skin irritation, and efficacy testing to satisfy regulators.
Documentation and Traceability: Maintaining detailed production records, batch traceability, and quality assurance documents.
Labeling and Packaging Support: Designing compliant labels with required safety information and ingredient declarations.
Regulatory Submissions Assistance: Helping with dossier preparation and registration processes.
Post-Market Surveillance: Supporting adverse event monitoring and reporting.
4️⃣ Custom Transdermal Capsicum Patches: Balancing Innovation with Compliance
Customization allows brands to differentiate through formulation and design, but it adds complexity to regulatory adherence.
OEM partners help by:
Advising on safe capsaicin concentrations and ingredient combinations to avoid regulatory conflicts.
Conducting compatibility and safety testing for new formulations.
Developing innovative patch designs (size, shape, adhesives) that meet safety and performance criteria.
Ensuring custom packaging and labeling fulfill country-specific requirements.
5️⃣ Private Label Transdermal Capsicum Patches: Compliance Considerations for Brand Owners
Brands opting for Private Label Transdermal Capsicum Patches must ensure their chosen OEM and supplier uphold compliance:
Verify the manufacturer’s certifications and quality systems.
Understand the regulatory classification of the product in target markets.
Confirm that labeling meets local legal requirements.
Monitor the supply chain for consistent quality and safety.
Collaborate on pharmacovigilance and adverse event reporting obligations.
6️⃣ Ensuring Quality and Safety Through the Supply Chain
Your Transdermal Capsicum Patches Supplier must implement rigorous controls:
Raw Material Verification: Capsaicin and excipients tested for purity and contaminants.
Production Environment: Controlled clean rooms and contamination prevention.
Batch Testing: Regular testing for potency, uniformity, and stability.
Packaging Integrity: Tamper-evident, hygienic packaging solutions.
Traceability: Complete lot-to-lot tracking for recall readiness.
7️⃣ How to Choose a Compliant Transdermal Capsicum Patches Manufacturer and OEM Partner
Selecting the right partner ensures compliance and smooth market access:
✔️ Certifications
Look for GMP, ISO 13485, FDA registration, CE marking capabilities, and other relevant certifications.
✔️ Regulatory Expertise
Assess their knowledge of international regulations and experience handling registration submissions.
✔️ Testing Facilities
On-site labs for stability, microbial, and safety testing increase turnaround time and quality control.
✔️ Transparency and Communication
Regular updates and documentation sharing build trust and reduce risks.
✔️ Customization & Packaging Capabilities
Ability to produce compliant custom formulas and packaging for multiple markets.
8️⃣ The Impact of Compliance on Brand Success
Brands that prioritize regulatory compliance through trusted OEM partnerships benefit from:
Faster Market Approvals: Smooth product launches across regions.
Reduced Legal Risks: Avoid fines, recalls, and reputational damage.
Consumer Confidence: Enhanced trust leading to higher sales and repeat business.
Global Reach: Ability to expand into lucrative international markets.
Sustainable Growth: Reliable supply chains and quality assurance enable long-term success.
9️⃣ Conclusion: Regulatory Compliance Is Non-Negotiable for Transdermal Capsicum Patches OEM
In an increasingly regulated global marketplace, ensuring Transdermal Capsicum Patches comply with all applicable standards is essential for your brand’s success. Partnering with a knowledgeable and certified Transdermal Capsicum Patches Manufacturer and Supplier through OEM arrangements enables you to navigate complex regulations confidently.
By leveraging their expertise in Custom Transdermal Capsicum Patches and Private Label Transdermal Capsicum Patches, you ensure your products are safe, effective, and ready for international distribution — safeguarding your brand and maximizing growth potential.
Related Questions and Answers
Q1: What certifications should a Transdermal Capsicum Patches Manufacturer have?
Look for GMP, FDA registration, CE marking, ISO 13485, and relevant local regulatory approvals.
Q2: Why is GMP important for Transdermal Capsicum Patches OEM?
GMP ensures consistent quality, safety, and compliance throughout production, minimizing risks.
Q3: Can customization affect regulatory compliance?
Yes, custom formulations and packaging must be tested and approved to meet all regulatory requirements.
Q4: What role does labeling play in compliance?
Labels must clearly state ingredients, usage instructions, warnings, and comply with market-specific regulations.
Q5: How do I ensure my Private Label Transdermal Capsicum Patches are compliant?
Partner with a reputable OEM, verify certifications, and review regulatory requirements for each target market.
Q6: Are adverse event reporting and post-market surveillance necessary?
Yes, ongoing monitoring is often mandatory to ensure product safety after launch.
Q7: How can I verify my Transdermal Capsicum Patches Supplier’s compliance?
Request certificates, audit reports, and regulatory documentation.
Q8: What challenges exist in global compliance for these patches?
Diverse classification systems, labeling standards, and ingredient restrictions across regions require careful management.






