How Do I Ensure That My Hot Capsicum Plasters Comply with Regulations?
When creating and selling Hot Capsicum Plasters, regulatory compliance is crucial for ensuring the safety, efficacy, and legality of your product in different markets. Whether you are working with a Hot Capsicum Plaster Manufacturer, a Hot Capsicum Plaster OEM, or developing a Custom Hot Capsicum Plaster, compliance with relevant health, safety, and labeling regulations should be a top priority. Non-compliance can lead to penalties, product recalls, or even legal challenges, which can be devastating for your brand. This article will guide you through the key steps required to ensure that your Hot Capsicum Plasters meet regulatory standards across the markets where you plan to distribute them.
1. Understanding the Regulatory Environment for Hot Capsicum Plasters
Before starting production or marketing your Custom Hot Capsicum Plaster, it is essential to understand the regulatory landscape in the regions where you intend to sell the product. Regulations can vary by country, with different requirements for product formulation, manufacturing practices, labeling, and claims.
For instance, in the United States, Hot Capsicum Plasters may fall under the purview of the Food and Drug Administration (FDA) as over-the-counter (OTC) drugs. In the European Union, they may be classified as medical devices, requiring compliance with the Medical Device Regulation (MDR). Other countries, such as Australia or Japan, have their respective regulatory bodies and guidelines.
By working with an experienced Hot Capsicum Plaster OEM, you can benefit from their knowledge of regional regulatory requirements, which will help streamline the compliance process.
2. Partnering with a Compliant Hot Capsicum Plaster Manufacturer or OEM
Choosing a Hot Capsicum Plaster Manufacturer that adheres to Good Manufacturing Practices (GMP) and other relevant standards is essential for ensuring your product's compliance. GMP certification guarantees that the manufacturing processes meet strict quality and safety standards. When working with a Hot Capsicum Plaster OEM, verify that they are certified and experienced in producing compliant products for your target market.
Here are some key factors to consider when selecting a Hot Capsicum Plaster Manufacturer:
GMP Certification: Ensures that the manufacturer follows standardized procedures for production, quality control, and safety.
ISO Certification: Compliance with ISO standards, such as ISO 13485, is often required for medical devices in certain markets like the EU.
Experience with Regulatory Compliance: The manufacturer should have experience producing Private Label Hot Capsicum Plasters that meet regulatory standards in various countries.
Testing and Documentation: Your OEM or manufacturer should have robust quality testing procedures and be able to provide documentation such as Certificates of Analysis (COA), product safety reports, and material safety data sheets (MSDS).
3. Labeling Requirements for Hot Capsicum Plasters
Accurate and compliant labeling is crucial for the sale of Hot Capsicum Plasters in any market. Labeling regulations ensure that consumers have access to necessary information about the product, including instructions for use, potential side effects, and safety warnings.
Your Custom Hot Capsicum Plaster label should include:
Product Name: Clearly state the product name as Hot Capsicum Plaster, along with any specific formulation or customization you’ve made.
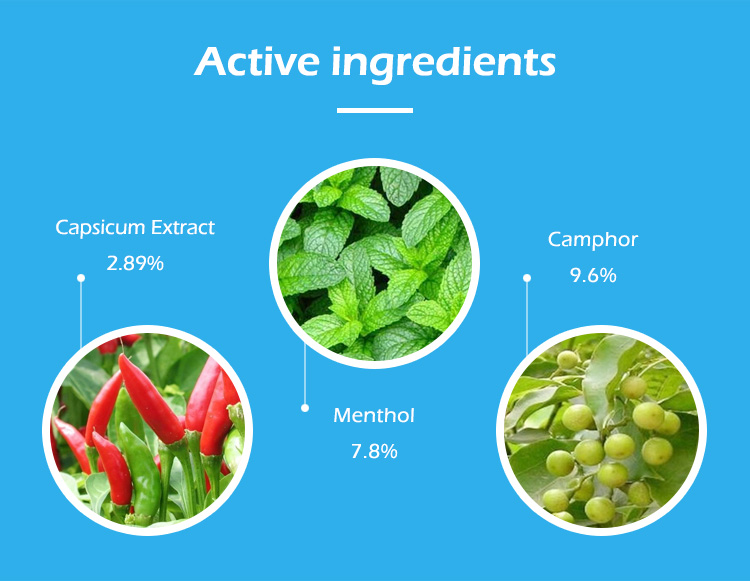
Active Ingredients: Capsicum is the key ingredient in Hot Capsicum Plasters, and its concentration should be clearly listed. Other active ingredients such as menthol or camphor must also be declared, with the respective percentages or dosages.
Usage Instructions: Provide detailed instructions on how to use the plaster, including how long it should remain on the skin, how frequently it can be applied, and any specific conditions it targets (e.g., muscle pain, arthritis, etc.).
Warnings and Contraindications: Include potential side effects, warnings for individuals with sensitive skin, and advice to discontinue use if irritation occurs. It's also important to mention contraindications, such as advising pregnant or breastfeeding women to consult with their doctor before using the product.
Storage Instructions: Specify how to store the Hot Capsicum Plasters, such as keeping them in a cool, dry place away from direct sunlight.
Manufacturing Information: Include details about the Hot Capsicum Plaster Manufacturer, such as the company’s name, address, and contact information, as well as any relevant certifications or batch numbers.
By working with a Hot Capsicum Plaster Supplier who understands the local regulations, you can ensure that your labeling is fully compliant with all applicable standards.
4. Ingredient Regulations and Safety Testing
Capsicum extract, the active ingredient in Hot Capsicum Plasters, is derived from chili peppers and is known for its heat-inducing, pain-relieving properties. While generally safe for topical use, the concentration of capsicum in your Custom Hot Capsicum Plaster must fall within acceptable safety limits. Regulatory authorities in various regions may have different guidelines regarding maximum concentrations for topical applications.
In addition to capsicum, other ingredients such as adhesives, preservatives, and enhancers should also be evaluated for safety and compliance. Depending on the market, certain chemicals may be restricted or banned.
Your Hot Capsicum Plaster OEM should perform comprehensive safety testing, which may include:
Skin Irritation and Sensitivity Testing: Ensures that the product is safe for use on various skin types and will not cause excessive irritation.
Toxicology Testing: Verifies that all ingredients are non-toxic when applied to the skin in the recommended amounts.
Stability Testing: Determines the shelf life of your Hot Capsicum Plaster and ensures that the active ingredients remain effective over time.
By conducting thorough testing and working with a knowledgeable Hot Capsicum Plaster Supplier, you can mitigate the risk of non-compliance and ensure the safety of your product.
5. Claims and Advertising Compliance
One of the most common regulatory pitfalls in the health and wellness industry is making unsubstantiated claims about a product’s efficacy. When marketing your Private Label Hot Capsicum Plaster, any claims regarding pain relief, healing properties, or therapeutic benefits must be backed by clinical evidence.
For example, if you claim that your Custom Hot Capsicum Plaster "relieves muscle pain for 12 hours," you must have data to support that statement. Regulatory bodies like the FDA (in the U.S.) and the MHRA (in the U.K.) actively monitor product claims to ensure they are truthful and not misleading.
Additionally, avoid using phrases like "cures" or "prevents diseases" unless you have appropriate approvals or evidence. Working with a Hot Capsicum Plaster OEM experienced in regulatory compliance can help ensure that your product claims are accurate and compliant.
6. Import and Export Compliance
If you plan to distribute your Hot Capsicum Plasters internationally, you must comply with the import and export regulations of each country. Many countries require detailed documentation before allowing medical or therapeutic products to be imported, including:
Product Registration: Some countries require registration of your Hot Capsicum Plaster with the national regulatory authority.
Certificates of Free Sale: These documents may be required to prove that your product is approved for sale in your home country.
Customs Documentation: Ensure that your Private Label Hot Capsicum Plaster is properly classified for import/export purposes, and all duties or taxes are paid.
Language Requirements: Labeling and instructions may need to be translated into the local language of the target market, ensuring full compliance with local regulations.
Conclusion
Ensuring that your Hot Capsicum Plasters comply with regulations is a multi-step process that requires careful attention to detail. From working with a certified Hot Capsicum Plaster Manufacturer or OEM, to performing safety testing, to ensuring accurate and compliant labeling, each stage of the process is vital for regulatory success. By following these steps and collaborating with knowledgeable partners, you can confidently bring your Custom Hot Capsicum Plaster to market while avoiding costly regulatory issues.
Related Questions and Answers:
What regulations should I consider when creating a Custom Hot Capsicum Plaster?
- You must adhere to regional regulations concerning ingredient safety, labeling, and product claims. Consult with a knowledgeable OEM to ensure compliance.
How can a Hot Capsicum Plaster OEM help with regulatory compliance?
- A qualified OEM can guide you through the regulatory landscape, ensuring your product meets safety, manufacturing, and labeling standards.
What should be included on the label of a Hot Capsicum Plaster?
- The label should include the product name, active ingredients, usage instructions, safety warnings, manufacturing details, and any applicable certifications.
Are there safety testing requirements for Hot Capsicum Plasters?
- Yes, your product should undergo skin irritation, toxicology, and stability testing to ensure it is safe for consumer use.
What are the key benefits of working with a compliant Hot Capsicum Plaster Manufacturer?
- Working with a compliant manufacturer ensures product safety, regulatory adherence, and streamlined entry into global markets.






